PURPOSE
The aims of this experiment are determining
magnesium by direct titration and determining calcium by displacement titration
and back titration.
THEORY
A complexation reaction involves a reaction
between a metal ion (M) and another molecular or ionic entity (D), containing
at least one atom with an unshared pair of electrons. Solvent or electron pair
donors occupy that all the coordination positions on metal ion.
Ligends are
called unidentate when they can donate one pair of electron such as ammonia and
multidentate when they can donate two or more electron pairs. The component is
called chelate when a multidentate ligand forms two or more coordinate bonds
with the same atom. A chelating agent bidentate, has two donor groups
availablefor coordination bonding and tridentate has three groups.
Tetradentate, pentadentate and hexadentate chelating agents are also known.
Chelation process occurs in single step. Equilibrium constants in complexation
reactions usually are expressed as formation or stability constats. Formation
constants generally are larger than those involving unidentate ligands. Halides
for analysis of mercury (II) and cyanide for analysis of Ag+ are
some of the common unidentate ligands. Ethylene diamine tetraacetic acid (EDTA)
is one of the most important polydentate ligands in analysis.
This hexadentate ligand forms very stable complexes (usually octahedral structures) with most of the transition metals.
One of the valuable properties of EDTA as a
titrant, is that it combines with metal ions (except alkali metals) in 1:1
ratio regardless of the charge on the cation.
pH is important for M-EDTA formation. For
example an alkaline medium is needed for titrations that involve Ca2+
and Mg2+, which form weak complexes. However some titrations require
moderately acidic solutions to form more stable complexes such as Zn2+
and Ni2+.
End point can be seen with using a metal ion
indicator. Eriochrome Black T (H3In) is the common one.
There are four titration methods;
1. Direct
titration: Determination
with EDTA, using metal ion indicators for end point detection.
2. Back
titration: It is useful
for the analysis of cations, that form very stable EDTA complexes and for which
a satisfactory indicator is not available.
3. Displacement
titration: It is useful for
introducing excess of a solution containing EDTA in the form of a Mg or Zn
complex.
4. Alcalimetric
titration: In this procedure, an
excess of Na2H2Y is added to a neutral solution of the
metal ion.
Reference
http://www.chem.purdue.edu/gchelp/cchem/edtalew.gif

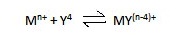

No comments:
Post a Comment